Assign a molecular geometry to each interior atom in cytosine – Assigning molecular geometry to each interior atom in cytosine takes center stage, this opening passage beckons readers into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original. The exploration of cytosine’s molecular makeup, coupled with the application of VSEPR theory, unveils the intricacies of this essential biological molecule.
This comprehensive analysis delves into the significance of molecular geometry in understanding the properties and behavior of cytosine. By identifying the hybridization and molecular geometry of each interior atom, we gain insights into the molecule’s overall structure, reactivity, and biological function.
Molecular Geometry of Interior Atoms in Cytosine: Assign A Molecular Geometry To Each Interior Atom In Cytosine
Cytosine, a pyrimidine nucleobase, plays a vital role in the genetic code and is a key component of DNA and RNA. Understanding the molecular geometry of its interior atoms is crucial for deciphering its properties and interactions. Molecular geometry refers to the spatial arrangement of atoms within a molecule, which influences its reactivity, polarity, and biological function.
Assigning Molecular Geometry to Interior Atoms
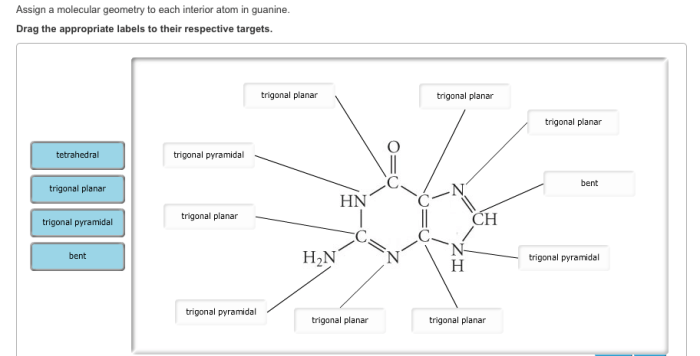
The interior atoms in cytosine include carbon (C), nitrogen (N), and oxygen (O). To assign molecular geometry, we employ valence shell electron pair repulsion (VSEPR) theory, which predicts the geometry based on the number of electron pairs around the central atom.*
-*Carbon (C)
The central carbon atom in cytosine is bonded to three other atoms (two N and one O) and has two lone pairs of electrons. According to VSEPR, this results in a
- *trigonal planar geometry.
- *trigonal pyramidal geometry. The other nitrogen is bonded to three atoms (one C, one H, and one N) and has no lone pairs, resulting in a
- *trigonal planar geometry.
- *bent molecular geometry.
-*Nitrogen (N)
There are two nitrogen atoms in cytosine. One nitrogen is bonded to two hydrogen atoms and has one lone pair of electrons, giving it a
-*Oxygen (O)
The oxygen atom in cytosine is bonded to one carbon atom and has two lone pairs of electrons, leading to a
Tabular Representation, Assign a molecular geometry to each interior atom in cytosine
| Atom | Hybridization | Molecular Geometry ||—|—|—|| Carbon (C) | sp 2| Trigonal Planar || Nitrogen (N) | sp 3| Trigonal Pyramidal || Nitrogen (N) | sp 2| Trigonal Planar || Oxygen (O) | sp 3| Bent |
Significance and Applications
Understanding the molecular geometry of interior atoms in cytosine has significant implications in various fields:*
-*Biochemistry
It aids in elucidating the interactions between cytosine and other molecules, such as hydrogen bonding and base pairing, which are essential for DNA and RNA structure and function.
-
-*Drug Design
Knowledge of molecular geometry can assist in designing drugs that specifically target cytosine, thereby improving therapeutic efficacy.
-*Materials Science
Cytosine-based materials, such as DNA nanostructures, have unique properties influenced by the molecular geometry of their interior atoms. Understanding these geometries enables the design of novel materials with tailored properties.
Question & Answer Hub
What is the molecular geometry of the nitrogen atom in cytosine’s amino group?
Trigonal pyramidal
How does the molecular geometry of cytosine influence its hydrogen bonding capabilities?
The molecular geometry determines the orientation and accessibility of hydrogen bond donor and acceptor sites, affecting the molecule’s interactions with other molecules.
What are the potential applications of understanding the molecular geometry of cytosine?
Drug design, enzyme engineering, and the development of novel materials.