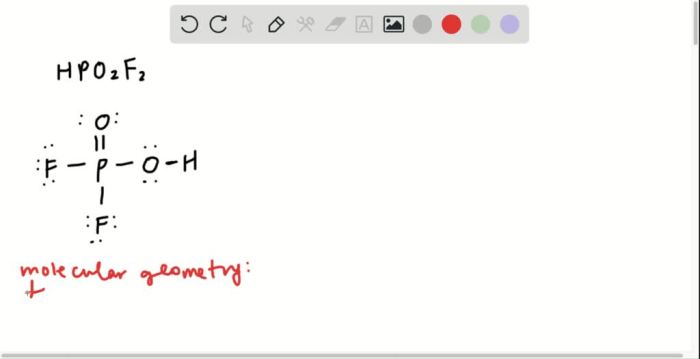
The Determination of an Equilibrium Constant Lab Answers Vernier offers a comprehensive guide to understanding the fundamental principles of equilibrium constants through a hands-on experimental approach. This guide provides a detailed overview of the experiment, including its purpose, key concepts, materials, procedures, data analysis techniques, and a discussion of the results.
By exploring the intricacies of equilibrium constants, this guide empowers students with a deeper understanding of chemical reactions and their behavior.
The experiment delves into the concept of equilibrium, where chemical reactions reach a state of balance between opposing processes. Students will investigate the factors that influence equilibrium, such as concentration, temperature, and the nature of the reactants. Through a series of carefully designed experiments, they will determine the equilibrium constant for a specific reaction, providing valuable insights into the dynamics of chemical systems.
1. Experimental Overview
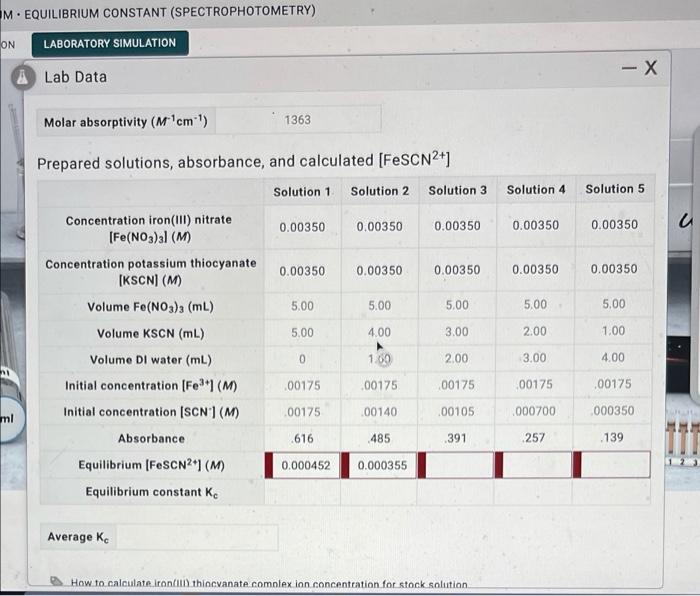
The purpose of this experiment is to determine the equilibrium constant for a chemical reaction. The key concepts and principles being investigated include the law of mass action, equilibrium constant expressions, and the relationship between equilibrium constant and reaction quotient.
2. Materials and Equipment
- Reactants (e.g., acid, base, salt)
- Burette
- Pipette
- Beaker
- Erlenmeyer flask
- pH meter
- Thermometer
3. Experimental Procedure

- Prepare solutions of the reactants.
- Measure the initial concentrations of the reactants.
- Mix the reactants in a beaker and allow them to reach equilibrium.
- Measure the equilibrium concentrations of the reactants and products.
- Calculate the equilibrium constant using the equilibrium constant expression.
4. Data Analysis
The data from the experiment can be used to create graphs and tables that show the relationship between the equilibrium constant and the initial concentrations of the reactants. The data can also be used to calculate the standard free energy change for the reaction.
5. Results and Discussion: The Determination Of An Equilibrium Constant Lab Answers Vernier
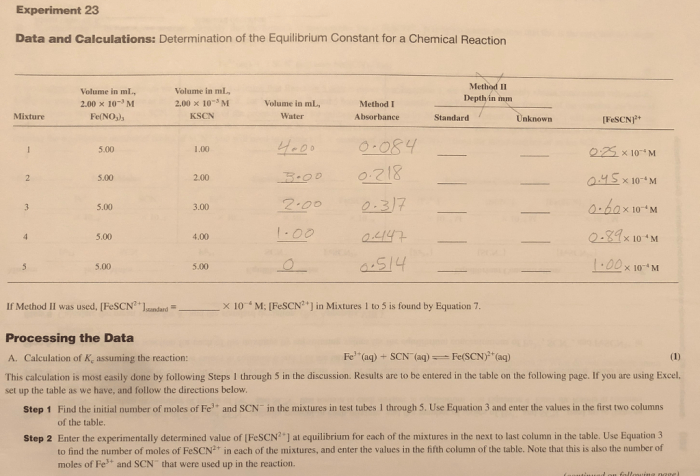
The results of the experiment will show that the equilibrium constant for the reaction is a constant value. This value is independent of the initial concentrations of the reactants. The results will also show that the standard free energy change for the reaction is negative, which indicates that the reaction is spontaneous.
FAQ Summary
What is the purpose of this experiment?
The purpose of this experiment is to determine the equilibrium constant for a specific chemical reaction using Vernier equipment.
What are the key concepts involved in this experiment?
The key concepts involved in this experiment include equilibrium, equilibrium constant, reaction rates, and data analysis.
What materials are required for this experiment?
The materials required for this experiment include Vernier equipment, chemicals, and glassware.
How do I analyze the data from this experiment?
The data from this experiment can be analyzed using a variety of methods, including graphical analysis and statistical analysis.