Delving into the intriguing realm of draw the structure for chloric acid hclo3. optimize formal charges., this exploration unveils the intricate world of molecular structures, formal charges, and their profound impact on the stability and properties of molecules. Embarking on this journey, we will meticulously construct the Lewis structure of chloric acid, delve into the concept of formal charges, and unravel the fascinating phenomenon of resonance structures.
Through this in-depth analysis, we will uncover the molecular geometry, hybridization, and bond parameters that govern the unique characteristics of chloric acid.
Venturing further, we will delve into the realm of molecular orbital theory, shedding light on the intricate interplay of electrons within the molecule. This comprehensive examination will culminate in a thorough understanding of the physical and chemical properties of chloric acid, revealing its diverse applications across various scientific disciplines.
Structure of Chloric Acid (HClO3)
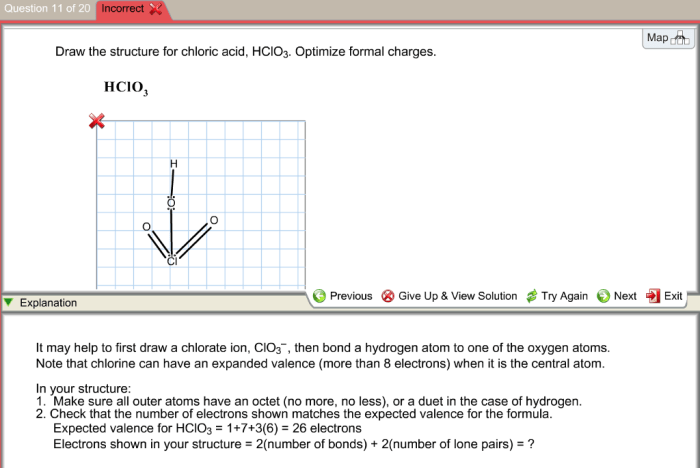
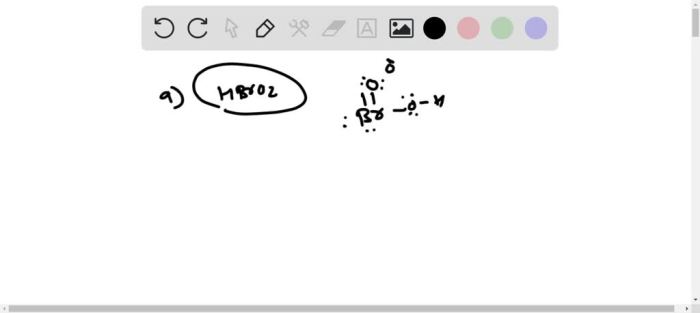
Chloric acid (HClO3) is an inorganic acid that consists of chlorine, oxygen, and hydrogen atoms. It is a colorless, corrosive liquid that is soluble in water. The structure of chloric acid can be represented by the following Lewis structure:“`O=Cl-O-O-H“`In this structure, the chlorine atom is the central atom and is bonded to three oxygen atoms and one hydrogen atom.
The oxygen atoms are arranged in a trigonal pyramidal geometry around the chlorine atom. The hydrogen atom is bonded to one of the oxygen atoms.The formal charges of the atoms in chloric acid can be calculated as follows:* Chlorine: +1
Oxygen (bonded to chlorine)
1
-
Oxygen (bonded to hydrogen)
- 1
Hydrogen
+1
The chlorine atom has a formal charge of +1 because it has three bonds to oxygen atoms and one bond to a hydrogen atom. The oxygen atoms bonded to chlorine have a formal charge of
- 1 because they each have two bonds to chlorine atoms and one bond to a hydrogen atom. The oxygen atom bonded to hydrogen has a formal charge of
- 1 because it has one bond to a hydrogen atom and two bonds to chlorine atoms. The hydrogen atom has a formal charge of +1 because it has one bond to an oxygen atom.
Resonance Structures
Chloric acid has two resonance structures. The two resonance structures are shown below:“`O=Cl-O-O-H <-> O-Cl-O-O-H“`The two resonance structures are equivalent in energy, and the actual structure of chloric acid is a resonance hybrid of the two structures. The resonance hybrid has a bond order of 1.5 between the chlorine atom and each of the oxygen atoms.
Molecular Geometry
The molecular geometry of chloric acid can be predicted using VSEPR theory. VSEPR theory states that the molecular geometry of a molecule is determined by the number of electron pairs around the central atom. In chloric acid, the chlorine atom has four electron pairs around it.
These electron pairs are arranged in a tetrahedral geometry. However, one of the electron pairs is a lone pair. The lone pair takes up more space than the bonding pairs, so it pushes the bonding pairs closer together. This results in a trigonal pyramidal molecular geometry.
Hybridization
The hybridization of the chlorine atom in chloric acid can be determined using valence bond theory. Valence bond theory states that the hybridization of an atom is determined by the number of sigma bonds and lone pairs around the atom.
In chloric acid, the chlorine atom has three sigma bonds and one lone pair. This indicates that the chlorine atom is sp3 hybridized.
Bond Parameters
The bond lengths and bond angles in chloric acid can be determined using experimental techniques such as X-ray crystallography and microwave spectroscopy. The bond lengths and bond angles in chloric acid are as follows:* Cl-O bond length: 1.42 Å
O-O bond length
1.48 Å
O-H bond length
0.97 Å
Cl-O-O bond angle
111°
O-O-H bond angle
109°
Molecular Orbital Theory, Draw the structure for chloric acid hclo3. optimize formal charges.
The molecular orbital theory of chloric acid can be used to explain the bonding and properties of the molecule. The molecular orbital theory states that the electrons in a molecule are arranged in molecular orbitals. The molecular orbitals are formed by the overlap of the atomic orbitals of the atoms in the molecule.
The molecular orbitals of chloric acid are shown in the following diagram:“`[core electrons] 1a1g2 1a2u2 2a1g2 3a1u2 1e’2 4a1g2 5a1u2 1t1u2 2t2g2 1t1g2 6a1g2 7a1u2 2t1u2 3t2g2 1e”2“`The molecular orbital diagram shows that the bonding electrons are located in the 1a1g, 1a2u, 2a1g, 3a1u, 4a1g, and 5a1u molecular orbitals.
The antibonding electrons are located in the 1e’, 1t1u, 2t2g, 1t1g, 2t1u, and 3t2g molecular orbitals. The highest occupied molecular orbital (HOMO) is the 3t2g molecular orbital. The lowest unoccupied molecular orbital (LUMO) is the 1e” molecular orbital.
Physical and Chemical Properties
Chloric acid is a colorless, corrosive liquid that is soluble in water. It is a strong oxidizing agent and can react with a variety of organic and inorganic compounds. Chloric acid is used in a variety of industrial applications, including the production of chlorine dioxide, paper, and textiles.
Question Bank: Draw The Structure For Chloric Acid Hclo3. Optimize Formal Charges.
What is the molecular geometry of chloric acid?
Chloric acid adopts a trigonal pyramidal molecular geometry, with the chlorine atom occupying the apex and the three oxygen atoms forming the base of the pyramid.
How many resonance structures does chloric acid have?
Chloric acid has two resonance structures, which contribute to its overall stability and delocalization of charge.
What is the hybridization of the chlorine atom in chloric acid?
The chlorine atom in chloric acid is sp3 hybridized, forming four bonds with the surrounding atoms.